Research interests
Research in our group involves the strategies for reaction methodology development to stand on contemporary problems in reaction discovery, and targeted synthesis. In this context, we believe that transition-metal-free carbon-carbon and carbon-heteroatom bond-forming reactions are key and green technologies, which is anticipated to become more and more important in the future. Our main research areas are:
- Aryne Chemistry
- Asymmetric catalysis
- Donor-Acceptor Cyclopropanes
- N-Heterocyclic carbene organocatalysis
Our present research focuses on the development of transition-metal-free carbon-carbon and carbon-heteroatom bond-forming reactions, and their implementation in organic synthesis. Specifically, we employ aryne chemistry for the rapid synthesis of various 1,2-disubstituted arenes. We also use N-heterocyclic carbene (NHC) based organocatalysis for the enantioselective construction of heterocycles and carbocycles. In addition to the synthesis of the benzo-fused compounds and chiral heterocycles, evaluation of the biological activity of these molecules forms part of our research.
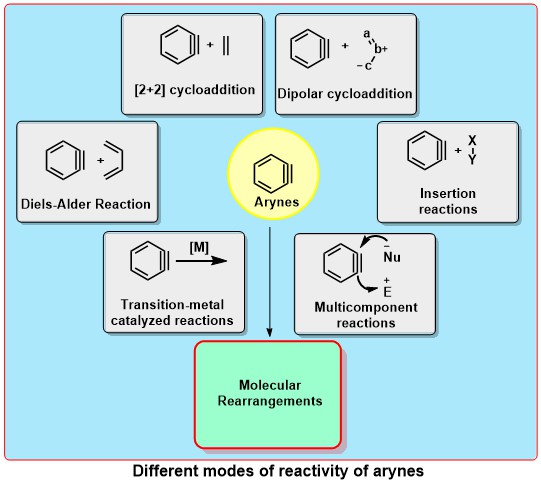
Transition-Metal-Free Aryne Reactions
Arynes are highly electrophilic reactive intermediates, which are widely used for the synthesis of 1,2-disubstituted arenes having molecular diversity and structural complexity. Although the chemistry of arynes is more than a century old, key to the resurgence of interest in the rich chemistry of arynes is primarily the mild condition for their generation by the fluoride-induced 1,2-elimination of 2-(trimethylsilyl)aryl triflates (Kobayashi protocol). The versatile transition-metal-free applications of arynes include cycloaddition reactions, insertion reactions, multicomponent coupling and molecular rearrangements. Developing new precursors for the mild generation of (het)arynes as well as employing arynes in enantioselective transformations appear to be the future goals in this area.
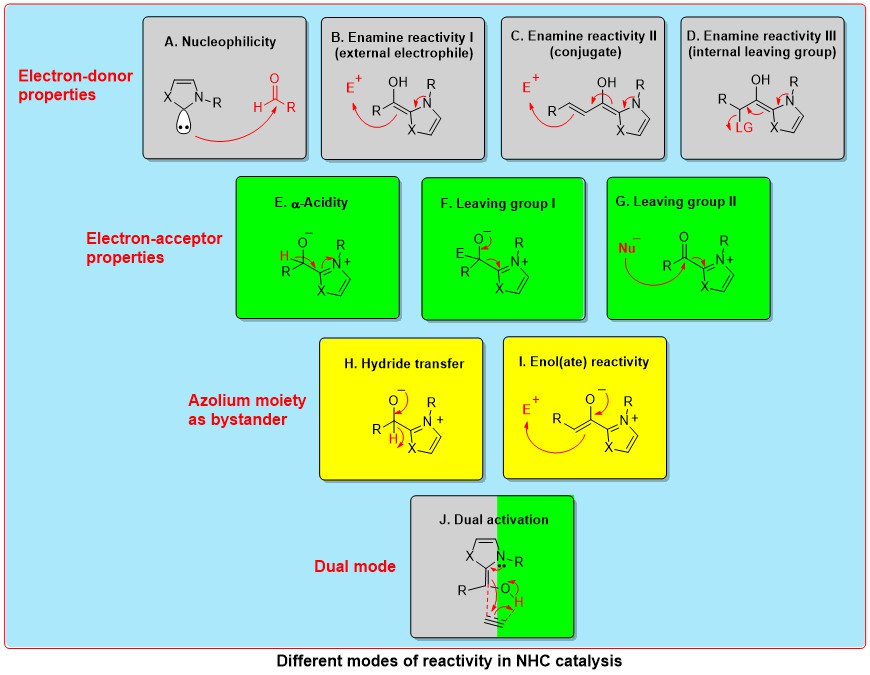
Sustainable N-Heterocyclic Carbene (NHC)-Organocatalysis
N-Heterocyclic carbene-catalysis has been used for the umpolung of aldehydes, and more recently to the umpolung of Michael acceptors and imines. The fundamental mode of carbene reactivity in organocatalysis is via the generation of nucleophilic Breslow intermediates (umpolung) by the addition of aldehydes to NHCs. The two well-known reactions proceeding via the umpolung of aldehydes are benzoin condensation and Stetter reaction. Moreover, the addition of enals to NHCs resulted in the generation of homoenolate equivalents (conjugate umpolung). In addition, NHCs are also known for catalyzing non-umpolung transformations. One such mode of carbene reactivity is via the generation of alpha, beta-unsaturated acyl azoliums. The concept of homoenolates and alpha, beta-unsaturated acyl azoliums has been utilized for the synthesis of various carbocycles, heterocycles and acyclic molecules. The use of chiral NHCs for these transformations can result in the synthesis of enantiomerically pure compounds. The important modes of NHC reactivity in catalysis are shown below.